
Founded: DNA Bank: 2006; Tissue Bank: 2017
Principal Investigator: Dr. Kubinyi Enikő
Funding: European Research Council (ERC), Hungarian Academy of Sciences National Brain Program
Contact: eniko.kubinyi@ttk.elte.hu
Overview
In 2024, we store DNA samples from 3800 canines, the brains and other samples (skin, muscle, liver, ganglion, blood, tumor) from 174 family dogs of various breeds, 5 wolves, and 19 jackals and ultra high resolution medical computed tomographic (UHR-CT) image series from 413 Canid and 18 Felid skulls. The tissue samples, collected within 4 hours post mortem, are fixed using three methods: (1) RNAlater, (2) flash-frozen, and (3) formaldehyde. The table below presents information on the available sample types and dog breeds (updated in 2022). If you are interested in collaborating, please contact eniko.kubinyi@ttk.elte.hu.
The Canine Tissue and Brain Bank (CBTB) was established following the protocols of human biobanks. Donating the bodies of euthanized pet dogs is a voluntary action based on the mutually agreed decision of the dogs' owners and the veterinarians who will perform the euthanasia. Both the owners and veterinarians are required to fill out a donation form, which verifies the dog's ownership and confirms that it had received valid rabies vaccination before the CBTB can accept the donation. Whenever the CBTB is informed about an impending donation, arrangements are made for the transportation of the cadavers prior to euthanasia to minimize the time between the animal's death and the fixation of tissue samples. As a result, we have been able to obtain molecular-grade tissue samples within four hours post mortem.
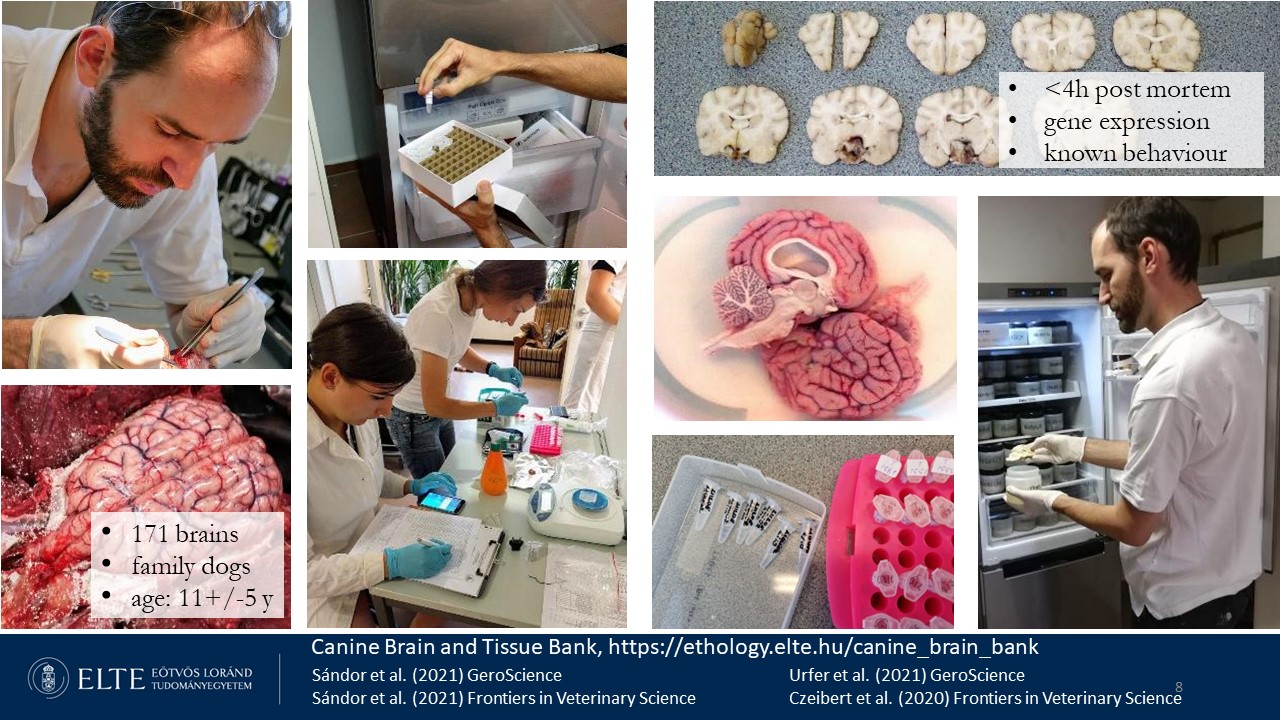
Samples
- Stabilized in RNAlater, Thermo Fisher: The locations from where we have collected RNAlater fixed tissue pieces from all animals (cases with <4 hours post mortem time) are the following: nose skin; skin from the head; temporal muscle; trigeminal ganglion (whole ganglion); prefrontal cortex (gyrus compositus anterior); cerebellum; brain stem. Samples are being stored at -80 °C for the long term.
- Furthermore, tissue pieces from some animals (<4 hours port mortem) from the following locations are also available: thyroid gland; retropharyngeal medial lymph node; liver and heart.
- We also collect and store liquor cerebrospinalis (CSF), when it is obtainable (e.g. the animals were not extensively dehydrated, in which cases the liquor has a very limited accessible volume).
- After taking tissue pieces from each target area and putting them in RNAlater, we process the whole brain: the right hemispheres get stored directly at -80 °C and the left hemispheres get fixed in 10% formaldehyde solution and stored at +4 °C.
- Buccal swabs and hair are also taken from all animals.
- In a few other cases, where the post mortem delay exceeds this limit, only the brains of the animals are collected and get stored primarily for histological purposes in formaldehyde.
The Canine Brain and Tissue Bank is introduced in details in Sándor et al. (2021), GeroScience.
The Canine Brain and Tissue Bank has been established within the framework of the Senior Family Dog Project (Department of Ethology, ELTE Eötvös Loránd University), to support its research goals and to set the fundaments of a repository of biological specimens, which could be distributed for other research groups on demand. It is located at Eötvös Loránd University, Budapest, Hungary, and utilizes the dissection room previously built for the Anatomy Department of the Biology Institute. All necessary instruments are available at the site or can be used in collaboration with other departments located in the same building. The core staff includes a veterinary anatomist, who performs the dissections, and two biologists, who participate in the sample management and are responsible to supervise subsequent steps in sample processing (e.g., removal of the RNAlater supernatant before deep freezing). Students or other researchers occasionally partake in the sampling events, providing support with simple tasks, like administration of sample IDs. Logistical and communication management (answering owners’ contact e-mails, making arrangements with veterinary clinics and owners, updating databases, etc.) is a shared responsibility of staff, depending on availability and schedule. Donations are accepted in every case when the necessary legislation criteria are fulfilled, and cases are subsequently categorized based on post-mortem interval, medical history, and other factors.
By 2023, the Canine Brain and Tissue Bank (CBTB) has collected brains and other tissues (e.g., skin from the head and temporal muscle) from more than 170 donated cadavers and actively supported current ongoing research projects, including international collaborations. So far, correlation between CKDN2A gene expression and age and ß-amyloid levels and age/cognitive function have been reported based on samples collected by the CBTB (see publications below). In the following, we will present the latest protocols and experiences of the CBTB.
Sample acquisition and quality
The quality of obtained tissue specimens is a major concern, especially in the case of brain banking. Neural tissue is particularly sensitive to environmental changes and starts to rapidly decompose after death. Several factors affect the quality of brain samples, such as post-mortem interval, pH value, and the grading of autolytic degradation of the granule cell layer. The acceptable range for these values depends in part on the research goals for which they are collected. DNA, for example, is more stable than RNA or most proteins. As the CBTB has been established to provide samples for a wide range of investigations related to the brain, including gene expression studies, the interval between the death of each animal and sample fixation was limited. Based on the human literature and practical considerations (towards conducting molecular research), the post-mortem interval for collecting brain tissue samples was set to 4 h in the CBTB. The developed donation system is allowed to maintain a narrow interval between the euthanasia of the animals and the fixation of samples in most cases. As the CBTB staff are in contact with most of the owners prior to the day of euthanasia, the timing of the donations can usually be planned in advance. When an animal gets euthanized in the prearranged time, a member of the CBTB with valid transport certificate arrives at the clinic and transports the cadaver to the facility of the CBTB. The dissection starts immediately after the arrival of the cadaver (usually 1–1.5 h post-mortem), meaning that on average, the brain tissue can be fixed within 2.5–3 h after the death of an animal.
Donations, for which the sampling time exceeded this limit, were considered for histological and anatomical research (e.g., brain cell counting) purposes only, and the brain tissues were fixed by phosphate-buffered saline-formaldehyde solution. Therefore, currently, two main forms of sampling protocol are performed by the CBTB, based on the elapsed time from the death of the animal. Protocol variant A was performed in donations with < 4 h post-mortem interval. Every other case underwent protocol B.
According to protocol A, brain regions and parts were distributed to gain both molecular grade and histological purpose samples from the same animals. First, the brains were halved in the midsagittal line. Tissue pieces from pre-defined areas of the brain were obtained from both hemispheres to allow the collection of corresponding probes from the same regions of each animal for methodological testing. To allow optimal penetration of the solution, an analytical scale was used to obtain 80–120 mg of each brain block to be immersed in 1 ml RNAlater, according to the manufacturer’s (Thermo Fisher Scientific) protocol. Although snap freezing is a common method used in human biobanking, RNAlater was chosen as a more convenient method for the stabilization of the relatively few standard tissue blocks obtained within the current, initial state of the CBTB protocol. Importantly, the RNAlater reagent was generally reported to provide reliable preservation of RNA content in tissues and cells. Also, RNAlater was shown to provide superior protection for samples in cases of freeze-thaw cycles. However, for the reliable preservation of larger/more numerous samples, snap freezing should be considered as the less expensive and more common method, which could be applied based on the examples of human brain banks.
In the currently applied protocol, the hemisphere intended for molecular purposes is divided into larger parts following anatomical borders (e.g., separating regions in the lines of common sulci). These parts are then frozen separately for later usage.
Another factor, which could be specifically relevant in canine biobanks, is the fact that most donated dogs are euthanized by chemical injections. It is known that chemicals (most commonly T-61) used to euthanize animals result in severe hemolysis and rapid changes in brain physiology before death. For this reason, blood samples from euthanized dogs were not considered for sensitive molecular research purposes in the CBTB and rather the pre-mortem collection of blood was inquired from the veterinarians when it was possible. However, it is also unclear how such changes would affect molecular components in the brain.
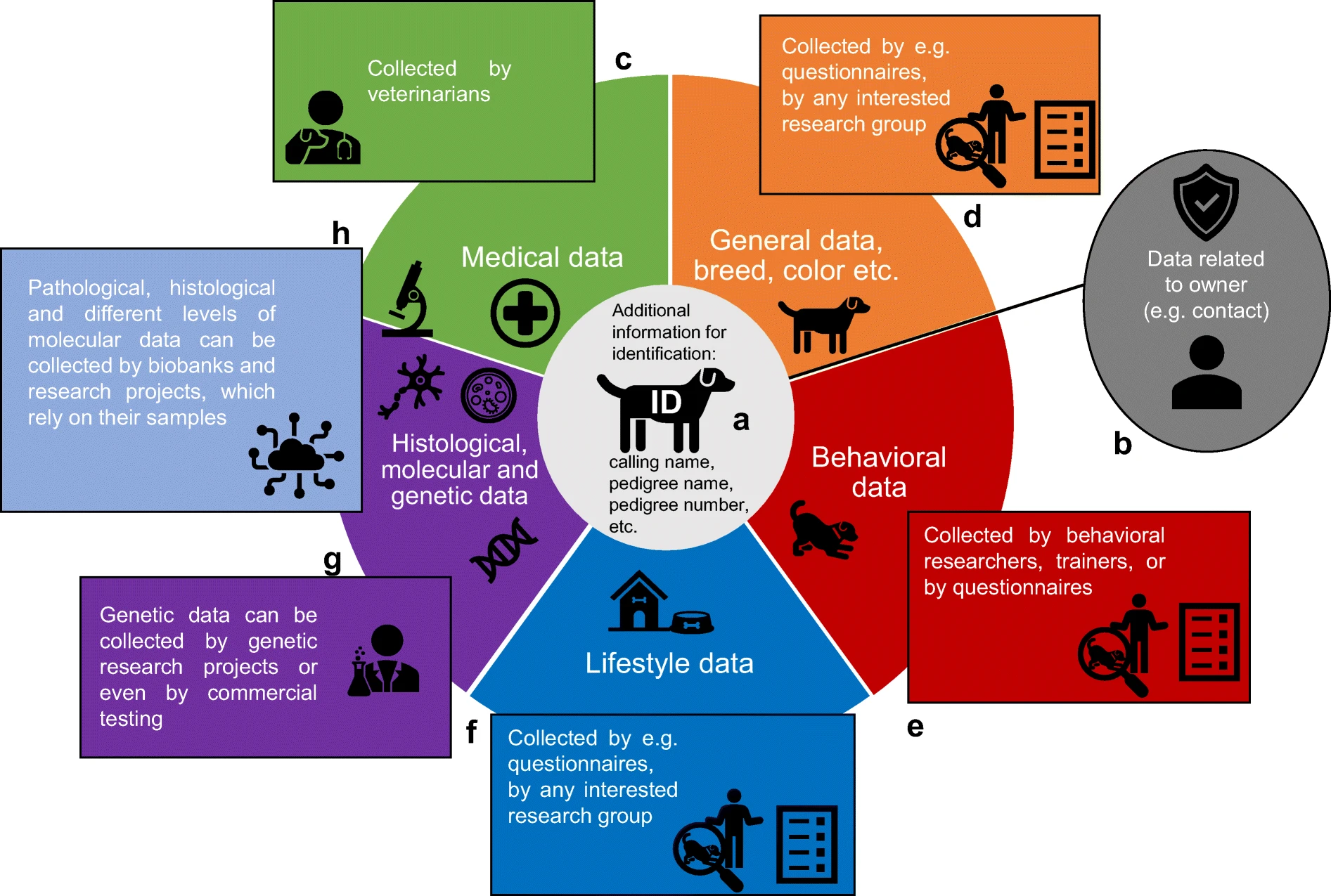 Data types, methods of data collection, and research groups that could be linked to animal biobank data. The CBTB aims to collect all types of data. Figure from Sándor et al. (2021), GeroScience.
Data types, methods of data collection, and research groups that could be linked to animal biobank data. The CBTB aims to collect all types of data. Figure from Sándor et al. (2021), GeroScience.
Collaboration with veterinarians and owners
In the case of the CBTB, the euthanasia is exclusively performed by collaborating veterinarians at veterinary clinics, which are located within a maximum of 1–1.5-h drive from the CBTB facility. Since the host institute does not include veterinary education and a related teaching clinic, permissions for veterinary care services and pet animal euthanasia are not available. As a second reason, in our experience, most owners choose to euthanize their pet dogs at a familiar clinic or at home and by a veterinarian whom they know and trust. Consequently, the protocol of the euthanasia may vary depending on the veterinarian who performs it according to individual professional experience and decision. However, all data about the chemical agents used for sedation and euthanasia are recorded on the consent form; thus, these factors can be controlled during subsequent analyses, if necessary. Apart from this inconsistency in the applied procedures and drugs, collaboration with third party veterinarians can have several advantages. Veterinarians performing the euthanasia have to officially state in the donation consent form that the euthanasia was justified by medical reasons and the dog did not suffer from medical conditions, which would be hazardous to the personnel performing the sample collection (e.g., rabies). Therefore, a veterinarian who had been familiar with the donated dog can provide a more detailed insight into the medical history of the donated dogs. Third-party veterinarians could also inform dog owners about the donation possibility, increasing the number of potentially informed owners. In turn, veterinarians—or owners—can receive feedback about the pathological state of the animal, which could be assessed during the dissection performed by the CBTB personnel, and samples can be sent for more detailed pathological examination on demand from the veterinarian or the owner.
The Canine Brain and Tissue Bank is open to national and international collaborations
This table contains information about the available sample types and dog breeds. We have RNAlater stabilized samples only from donations where the post-mortem delay was <4 hours.
| Tissue type / region | Storage method | Number of animals | Breeds | Other species | Remark |
| Whole brain (right hemisphere) | Simply frozen at -80°C as a whole (old sampling procedure) | 60 | Beagle(10), Bichon havanaise (2); Border Collie(3); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Yorkshire terrier(2); Mongrel (variable,20) | Quality of samples may be questionable in cases where pieces have already been cut out from the brain tissue. We keep track of freeze-thawing cycles. | |
| Whole brain (right hemisphere) | Sliced and parts frozen separately at -80°C (current method) | 18 | Beagle (2); Borzoi(1); Whippet(1); Mongrel(5); White Swiss Shepherd Dog (1); Vizsla (1); Gordon Setter (1); Groenendael (1); Collie (1); German Shepherd Dog (2);Labrador Retriever (1); Staffordshire Terrier (1) | ||
| Whole brain (or left hemisphere) | Whole hemisphere fixed in 4% formaldehyde, stored at 4°C | 135 | Beagle(13); Bichon bolognese(1); Bichon frise(1); Bichon havanaise(3); Bobtail(1); Border Collie(3); Borzoi(1); Boxer(5); Caucasian Shepherd Dog(1); Chihuanua(1); ; Dachshund(1); Dobermann(1); English Bulldog(2); English Cocker Spaniel (1); German Shepherd(2); Golden retriever(3); Gordon Setter(1); Labrador retriever(6); Leonberger (1); Miniature poodle (2); Mudi(1); Puli (2); Rottweiler(1); Small Münsterlander(1); Staffordshire Terrier (1); Vizsla(2); Westie (2); Whippet(1); Yorkshire terrier(5); Mongrel (variable, ~30) | Canis aureus (19) and Canis lupus (1)*, please ask for details | |
| Brain / frontal cortex | Whole brain simply frozen at -80°C (1th phase sampling) | 60 | Beagle(10), Bichon havanaise (2); Border Collie(3); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Yorkshire terrier(2); Mongrel (variable,20) | ||
| Brain / frontal cortex | Sliced and frozen at -80°C (current method) | 18 | Beagle (1); Beagle (2); Borzoi(1); Whippet(1); Mongrel(5); White Swiss Shepherd Dog (1); Vizsla (1); Gordon Setter (1); Groenendael (1); Collie (1); German Shepherd Dog (2);Labrador Retriever (1); Staffordshire Terrier (1) | ||
| Brain / frontal cortex | RNA later stabilized tissue piece | 64 | Beagle(11), Bichon havanaise (2); Border Collie(3); Borzoi(1); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Whippet(1); Yorkshire terrier(2); Mongrel (variable,21) female | Canis lupus (4), please ask for details | Some samples have already been used up for research, please ask for details. |
| Brain / frontal cortex | Fixed in 4 % formaldehyde as whole brain hemisphere | 135 | Beagle(13); Bichon bolognese(1); Bichon frise(1); Bichon havanaise(3); Bobtail(1); Border Collie(3); Borzoi(1); Boxer(5); Caucasian Shepherd Dog(1); Chihuanua(1); ; Dachshund(1); Dobermann(1); English Bulldog(2); English Cocker Spaniel (1); German Shepherd(2); Golden retriever(3); Gordon Setter(1); Labrador retriever(6); Leonberger (1); Miniature poodle (2); Mudi(1); Puli (2); Rottweiler(1); Small Münsterlander(1); Staffordshire Terrier (1); Vizsla(2); Westie (2); Whippet(1); Yorkshire terrier(5); Mongrel (variable, ~30) | Some samples have already been used up for research, please ask for details. | |
| Brain / cerebellum | Whole brain simply frozen at -80°C (1th phase sampling) | 60 | Beagle(10), Bichon havanaise (2); Border Collie(3); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Yorkshire terrier(2); Mongrel (variable,20) | ||
| Brain / cerebellum | Sliced and frozen at -80°C (current method) | 18 | Beagle (2); Borzoi(1); Whippet(1); Mongrel(5); White Swiss Shepherd Dog (1); Vizsla (1); Gordon Setter (1); Groenendael (1); Collie (1); German Shepherd Dog (2);Labrador Retriever (1); Staffordshire Terrier (1) | ||
| Brain / cerebellum | RNA later stabilized tissue piece | 64 | Beagle(11), Bichon havanaise (2); Border Collie(3); Borzoi(1); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Whippet(1); Yorkshire terrier(2); Mongrel (variable,21) female | Canis lupus (4), please ask for details | Some samples have already been used up for research, please ask for details. |
| Brain / cerebellum | Fixed in 4 % formaldehyde as whole brain hemisphere | 135 | Beagle(13); Bichon bolognese(1); Bichon frise(1); Bichon havanaise(3); Bobtail(1); Border Collie(3); Borzoi(1); Boxer(5); Caucasian Shepherd Dog(1); Chihuanua(1); ; Dachshund(1); Dobermann(1); English Bulldog(2); English Cocker Spaniel (1); German Shepherd(2); Golden retriever(3); Gordon Setter(1); Labrador retriever(6); Leonberger (1); Miniature poodle (2); Mudi(1); Puli (2); Rottweiler(1); Small Münsterlander(1); Staffordshire Terrier (1); Vizsla(2); Westie (2); Whippet(1); Yorkshire terrier(5); Mongrel (variable, ~30) | Some samples have already been used up for research, please ask for details. | |
| Brain / brain stem | Whole brain simply frozen at -80°C (1th phase sampling) | 60 | Beagle(10), Bichon havanaise (2); Border Collie(3); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Yorkshire terrier(2); Mongrel (variable,20) | ||
| Brain / brain stem | Sliced and frozen at -80°C (current method) | 25 | Beagle (2); Borzoi(1); Whippet(1); Mongrel(5); White Swiss Shepherd Dog (1); Vizsla (1); Gordon Setter (1); Groenendael (1); Collie (1); German Shepherd Dog (2);Labrador Retriever (1); Staffordshire Terrier (1) | ||
| Brain / brain stem | RNA later stabilized tissue piece | 64 |
Beagle(11), Bichon havanaise (2); Border Collie(3); Borzoi(1); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Whippet(1); Yorkshire terrier(2); Mongrel (variable,21) | Canis lupus (4), please ask for details | Some samples have already been used up for research, please ask for details. |
| Brain / brain stem | Fixed in 4 % formaldehyde as whole brain hemisphere | 135 | Beagle(13); Bichon bolognese(1); Bichon frise(1); Bichon havanaise(3); Bobtail(1); Border Collie(3); Borzoi(1); Boxer(5); Caucasian Shepherd Dog(1); Chihuanua(1); ; Dachshund(1); Dobermann(1); English Bulldog(2); English Cocker Spaniel (1); German Shepherd(2); Golden retriever(3); Gordon Setter(1); Labrador retriever(6); Leonberger (1); Miniature poodle (2); Mudi(1); Puli (2); Rottweiler(1); Small Münsterlander(1); Staffordshire Terrier (1); Vizsla(2); Westie (2); Whippet(1); Yorkshire terrier(5); Mongrel (variable, ~30) | Some samples have already been used up for research, please ask for details. | |
| Skin from head | RNA later stabilized tissue piece | 64 | Beagle(11), Bichon havanaise (2); Border Collie(3); Borzoi(1); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Whippet(1); Yorkshire terrier(2); Mongrel (variable,21) | ||
| Skin from nose | RNA later stabilized tissue piece | 64 | Beagle(11), Bichon havanaise (2); Border Collie(3); Borzoi(1); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Whippet(1); Yorkshire terrier(2); Mongrel (variable,21) | ||
| Musculus temporalis | RNA later stabilized tissue piece | 64 | Beagle(11), Bichon havanaise (2); Border Collie(3); Borzoi(1); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Whippet(1); Yorkshire terrier(2); Mongrel (variable,21) | Some samples have already been used up for research, please ask for details. | |
| Ggl. trigeminale | RNA later stabilized tissue piece | 64 | Beagle(11), Bichon havanaise (2); Border Collie(3); Borzoi(1); Boxer(1);Chihuanua(1); Dachshund(1);Dobermann(1); German Shepherd(1); Golden retriever(2); Gordon Setter(1); Vizsla(2); Labrador retriever(5);Miniature poodle (2); Puli (1); Small münsterlander(1); Whippet(1); Yorkshire terrier(2); Mongrel (variable,21) | ||
| Ggl. distale | RNA later stabilized tissue piece | 50 | Beagle (7); Border Collie (2); Bichon Havanaise (2); Boxer (1); Chihuahua (1); Dachshund(1); Dobermann(1); German Shepherd Dog (3); Golden Retriever (2); Gordon Setter (2); Labrador Retriever (4); Miniature poodle (2); Small Münsterlander (1); Vizsla (2); Yorkshire Terrier (2); Mongrel (12); White Swiss Shepherd Dog (1); Vizsla (1); Groenendael (1); Collie (1); Staffordshire Terrier (1) | Standard collection was discontinued from 2019. | |
| Ggl. Cervicale (C4 or C5*) | RNA later stabilized tissue piece | 50 | Beagle (7); Border Collie (2); Bichon Havanaise (2); Boxer (1); Chihuahua (1); Dachshund(1); Dobermann(1); German Shepherd Dog (3); Golden Retriever (2); Gordon Setter (2); Labrador Retriever (4); Miniature poodle (2); Small Münsterlander (1); Vizsla (2); Yorkshire Terrier (2); Mongrel (12); White Swiss Shepherd Dog (1); Vizsla (1); Groenendael (1); Collie (1); Staffordshire Terrier (1) | Standard collection was discontinued from 2019. | |
| Gl. thyreoidea | RNA later stabilized tissue piece | 50 | Beagle (7); Border Collie (2); Bichon Havanaise (2); Boxer (1); Chihuahua (1); Dachshund(1); Dobermann(1); German Shepherd Dog (3); Golden Retriever (2); Gordon Setter (2); Labrador Retriever (4); Miniature poodle (2); Small Münsterlander (1); Vizsla (2); Yorkshire Terrier (2); Mongrel (12); White Swiss Shepherd Dog (1); Vizsla (1); Groenendael (1); Collie (1); Staffordshire Terrier (1) | Standard collection was discontinued from 2019. | |
| Ln. retropharyngeus medialis | RNA later stabilized tissue piece | 50 | Beagle (7); Border Collie (2); Bichon Havanaise (2); Boxer (1); Chihuahua (1); Dachshund(1); Dobermann(1); German Shepherd Dog (3); Golden Retriever (2); Gordon Setter (2); Labrador Retriever (4); Miniature poodle (2); Small Münsterlander (1); Vizsla (2); Yorkshire Terrier (2); Mongrel (12); White Swiss Shepherd Dog (1); Vizsla (1); Groenendael (1); Collie (1); Staffordshire Terrier (1) | Standard collection was discontinued from 2019. | |
| Liver | RNA later stabilized tissue piece | 18 | Border Collie; Puli; Borzoi; Beagle; Vizsla; Wolf; Bull terrier; German shepherd dog (2);Labrador Retriever; Staffordshire Terrier; Mongrel (5) | Standard collection started from 2020. | |
| Tumors | RNA later stabilized tissue piece | Samples collected on ocassion, ask for more details! |
Publications
- Garamszegi, L., Kubinyi, E., Czeibert, K., Nagy, G., Csörgő, T., Kolm, N., Evolution of relative brain size in dogs - no effects of selection for breed function, litter size or longevity, Evolution (2023)
- Kovács, T., Szinyákovics, J., Billes, V., Murányi, G., Varga, V., Bjelik, A., Légrádi, Á., Szabó, M., Sándor, S., Kubinyi, E., Szekeres-Paracky, C., Szocsics, P., Lőke, J., Mulder, J., Gulyás, B., Renner, É., Palkovits, M., Gulya, K., Maglóczky, Z., Vellai, T., A conserved MTMR lipid phosphatase increasingly suppresses autophagy in brain neurons during aging, Scientific Reports (2022)
- Sándor, S., Urfer, S., Kubinyi, E., Toward establishing a worldwide net of canine biobanks, Aging-Us 14(6), 2436–2437. (2022)
- Sándor, S., Jónás, D., Tátrai, K., Czeibert, K., Kubinyi, E. Poly(A) RNA sequencing reveals age-related differences in the prefrontal cortex of dogs. GeroScience, 44, 1269–1293. https://doi.org/10.1007/s11357-022-00533-3 (2022)
- Urfer, S.R., Darvas, M., Czeibert, K., Sándor, S. Promislow, D. E. L., Creevy, K. E., Kubinyi*, E., Kaeberlein*, M. Canine Cognitive Dysfunction (CCD) scores correlate with amyloid beta 42 levels in dog brain tissue. GeroScience, 43:2379–2386. https://doi.org/10.1007/s11357-021-00422-1. *These authors jointly supervised this work. (2021)
- Sándor, S., Czeibert, K., Salamon, A., & Kubinyi, E. Man’s best friend in life and death: scientific perspectives and challenges of dog brain banking. GeroScience, 43:1653–1668. https://link.springer.com/article/10.1007/s11357-021-00373-7 (2021)
- Sándor, S., Tátrai, K., Czeibert, K., Egyed, B., & Kubinyi, E. (2021). CDKN2A gene expression as a potential aging biomarker in dogs. Frontiers in Veterinary Science, 8, 348. https://www.frontiersin.org/articles/10.3389/fvets.2021.660435/full, 10.3389/fvets.2021.660435
- Czeibert, K., Sommese, A., Petneházy, Ö., Csörgő, T., Kubinyi, E., Digital Endocasting in Comparative Canine Brain Morphology, Frontiers In Veterinary Science (2020)
